Figure Source
Finding Spectra on the Web
Before looking at specific spectra, I want to introduce a good source of finding IR spectra, so that the reader can find them on his/her own. The National Institute of Standards and Technology (NIST) has a resource on the web for finding information about chemicals called the NIST webbook.
This site allows one to search by several categories. For example one can search by chemical formula:

In this case, I searched for "CO2" and checked the appropriate boxes:
Scrolling down or clicking "IR Spectrum" yields the following:

Clicking on the link to the spectrum brings up a viewer as well as several options for downloading the spectrum or the spectral data. The viewer provides several options for looking at the data:
One can choose the x-axis to be in wavenumbers (cm-1) or microns. The relationship between these units is explained in a previous post "What is Infrared Radiation (IR)?" Neglecting the small vacuum correction, the conversion is simple. Convert microns to centimeters (There are 106 microns in a meter and 102 centimeters in a meter; so there are 104 microns in a centimeter), and take the reciprocal. One can choose to reverse the x-axis so that the smallest value in wavenumbers is on the left, or leave the units so that the smallest value in microns is on the left.
One can choose to view the spectrum as transmittance or as absorbance. The relationship between transmittance and absorbance is explained in a subsequent post about the Beer-Lambert Law. To convert between transmittance (τ) and Absorbance in the case of this spectrum one can use the following formulas:
τ = 10-A and A = -log(τ )
One has to be careful, however. Sometimes the relationship is expressed as log based 10 and sometimes as the natural log. It would be nice if researchers were consistent in following one standard or the other, but the truth is that they are not and one needs to make sure which standard is being used.
The spectrum of carbon dioxide can be viewed in any combination of these choices. For example, one could choose transmittance and wavenumbers:
One can also choose to see the spectrum in absorbance and microns:
The IR Spectrum of Carbon Dioxide
The feature at 4.2 microns is assigned to the asymmetric stretch mode. The feature at 15 microns is the bending mode. This feature is what all the fuss is about. The features arise from the rotational structure as explained in the previous post. This feature is between 600 and 750 cm-1 an energy range that overlaps considerably with the earth's black-body spectrum. The feature at 2.7 microns is a "forbidden" overtone band.
The Spectrum of Nitrous Oxide
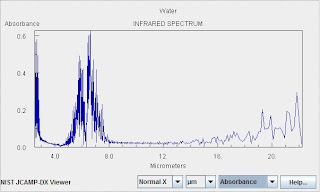
The Spectrum of Water Vapor
Water is an extremely important and also complicated greenhouse gas. Without the role of water vapor as a greenhouse gas, the earth would be uninhabitable. Water is not a driver or forcing in anthropogenic warming, however. Rather it is a feedback, and a rather complicated one at that. The amount of water vapor in the air changes in response to forcings (such as the solar cycle or warming owing to anthropogenic emission of carbon dioxide). This change in water vapor concentration leads to positive and negative feedback mechanisms because of its role as a greenhouse gas, but also because of the role of liquid and solid aerosols (clouds), the effect of on the heat capacity of the air, and the influence of water phase transitions.
As indicated above water vapor is extremely complicated. Water exhibits overtones and combination bands. In addition there is extensive coupling between vibrational and rotational degrees of freedom because the vibrations change the the moment of inertia. Collisional broadening of the "feet" of water lines leads to an atmospheric feature know as the water continuum.
In the HCl molecule, I briefly discussed spectroscopic splitting arising from the distribution of chlorine isotopes. In water, the case is much more extreme as the Deuterium isotope of hydrogen is twice the mass of the most common hydrogen isotope, and D2O has a very distinct spectrum.
Furthermore, water can form hydrogen bonded clusters, which add to the complication of the spectroscopy. Furthermore, along these lines water exists in all three phases in the atmosphere, liquid, solid, and vapor. These phases have spectra different from the vapor spectrum and water aerosols cause Mie scattering. Suffice it to say that the study of atmospheric water spectroscopy is a very rich field.
Ozone
Although Ozone in the troposphere exists at very small concentrations relative to carbon dioxide and water vapor, it still forms an important component of the IR spectrum of the troposphere because of its strong absorption peaks. Ozone has a symmetric stretch at about 9 microns, and asymmetric stretch at about 9.6 microns, and a bend at about 14 microns.
Methane
Methane is an important greenhouse gas. One of the extreme scenarios of global warming is the possibility that warming owing to carbon dioxide will cause the release of methane trapped in methane hydrate in the arctic. Such a process could cause a runaway positive feedback.
Methane has two allowed modes each of which is degenerate. They can be seen at about 3.3 microns and 7.7 microns.
The reader should now have the tools to understand some of the basics of how molecular structure gives rise to absorption and emission in the infrared region; the reader should be able to access the NIST database and search for molecular species of interest. The next post in this series starts the discussion of how to understand how much radiation is emitted or absorbed by molecules in the atmosphere.
Sources
- NIST Webbook
- Widener: CarbonDioxide
- Near IR Spectra of Carbon dioxide in H2O
- Infrared Intensities of Nitrous Oxide
- HITRAN Nitrous Oxide Data
- Characteristic Vibrations of N2O
- Characteristic Vibrations of H2O
- The Water Vapor Continuum
- VPL Molecular Spectroscopic Database
- The Infrared Absorption Spectrum and the Molecular Structure of Ozone
- The infrared spectrum and the molecular structure of ozone and sulfur dioxide
- Near infrared absorption spectra of ozone by high resolution Fourier transform spectrometry










No comments:
Post a Comment