The current post is intended to wrap up the topic and touch upon a few issues that were not discussed. It is possible to teach a year-long course in radiative transfer (or even multiple courses); so of course this post does not do the topic justice, but perhaps it provides some basic principles that give the reader a cursory understanding of the topic.
Radiation Incident on a Layer
By conservation of energy, three things can happen to radiation that is incident on a layer of the atmosphere. The radiation can be absorbed by the layer, the radiation can be transmitted by the layer or the radiation can be scattered by the layer. There are processes such as Raman scattering in which radiation can be absorbed and scattered (or absorbed, emitted, and scattered), but overall the percent of incident radiation that is absorbed, the percent that is transmitted, and the percent that is scattered must add up to 100%. A layer can also emit radiation, but this energy comes from energy within the layer, not from the incident energy.
Overall, energy must be conserved. Consider that the universe is composed of a layer of the atmosphere, and everything else. The layer constitutes a system of interest, and the surroundings represent the rest of the universe. The energy of the system plus the energy of the surroundings is constant. If more energy is transferred from the surroundings to the system than from the system to the surroundings, the energy of the system must increase and vice versa.
Transmission and Extinction
The energy that is scattered and the energy that is absorbed are sometimes referred to as extinction. The energy that is neither absorbed nor scattered is transmitted.
Absorption and Emission
The previous post in this series presented a multi-layer model of transmission and absorption of carbon dioxide in an earth-like system. The spectra used were relatively low resolution spectra obtained from NIST. Such low resolution spectra are inadequate when one is concerned with calculating the energy balance of the earth. Additionally, spectra change as a function of temperature and pressure and it is necessary to incorporate those changes into a model. At this time, I am not going to produce such a model, but many such models can be found in some of the references listed below.
The data in the HITRAN database can be used to create high resolution spectra of IR absorbers in such a manner that allows the spectrum to vary with the layer treated.
In addition to absorption and emission, it is also necessary to treat scattering.
Scattering
Because the speed of light within matter is different than the speed of light in vacuum, matter deflects or scatters incident light from its path. There are different regimes of scattering. The phenomenon of scattering can explain why the sky is blue, why clouds are white, and many other optical phenomena.
If the particles are much smaller than the wavelength of light being scattered, the scattering is in the Rayleigh regime, in which blue light is scattered more efficiently than red light. That is the reason that the sky is blue and sunsets are red. As the particles get bigger relative to the radiation, the scattering becomes Mie scattering, and even larger particles enter the regime of geometric optics.
Scattering from clouds (water and ice aerosols) and other aerosols (such as dust, volcanic ash etc.) is important to include in full scale climate models. Although water clouds have the net effect of absorbing IR radiation, they also scatter visible radiation from the sun.
Energy Balance
Estimating the effects of greenhouse gases all comes down to energy balance and applying the principle that energy is conserved. By integrating the high resolution IR spectrum of the atmosphere across layers and also across the spectrum (line-by-line integration by wavenumber), it is possible to estimate the power (energy per time) being absorbed by the atmosphere from the IR emission of the earth. With knowledge of the heat capacity of the atmosphere, the heating rate can be calculated. Future posts on this blog (but not in this series) will address energy balance and climate sensitivity.
Drivers
The problem of climate change can be simplified if one thinks about the major influences on the earth's temperature. These influences are twofold: forcing and feedback. A forcing can be thought of as an external driver to which the climate responds in some way. A feedback is a response to that forcing that also influences climate. There are really three major climate forcings: variation in the solar radiation incident on the earth, volcanoes, and greenhouse gases.
The Sun

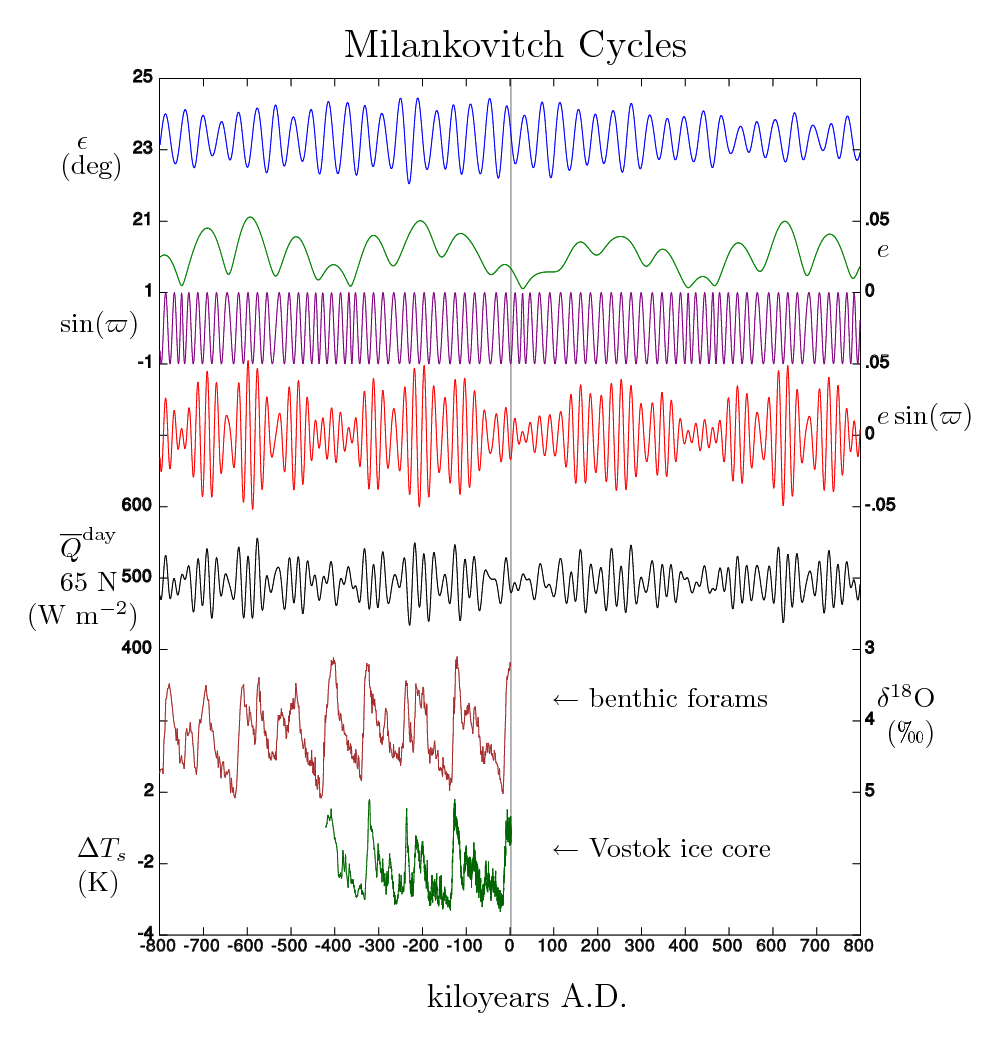
Obviously, the energy coming into the earth system from the sun is a crucial element to understanding the earth's energy balance. As of this writing (June 2010), the sun is coming out of a very pronounced minimum in its approximately 11-year cycle. In the next few years, we should expect the radiation incident upon the earth to increase. Over very long time scales orbital variation of the earth also changes the amount of radiation incident from the sun. These variations, called Milankovitch cycles, are though to be responsible for triggering the ice-ages, and initiating the end of ice ages.
Volcanoes
Volcanic eruption have a cooling effect on the earth as the ash clouds scatter incident solar radiation.
(Image Source)
Greenhouse Gases
The elephant in the room, in terms of climate forcing, is the role of anthropogenic emissions of greenhouse gases. Those who have been following this series should have an understanding of why they effect climate. Further explanation should be needed to understand how much and how fast and what this has to do with burning fossil fuels.
Other Forcing
There are other forcings that are less important, among these perhaps changes in the earth's albedo by human activity is probably the most significant.
Feedback
Water, Water, and Water
On timescales of interest, there are three major, feedbacks, all of which involve water: water vapor, cloud formation (to include ice), and changes in the earth's albedo from the meting of sea ice, the ice caps, and glaciers. Over time periods of interest, these feedbacks are generally estimated to be a positive feedback, e.g., they add to the effect of warming.
(Image source)
Other Feedback
Greenhouse gases can act as a feedback as well as a forcing. The natural cycles of carbon dioxide can be considered a feedback to other process that warm and cool the earth. The extreme scenario (sometimes called the clathrate gun hypothesis) that anthropogenic global warming could cause the release of methane from methane clathrates and that the released methane would cause runaway warming is an example of a greenhouse gas as a feedback.
Over very long periods of time there is a chemical process involving calcium ions in the ocean that is thought to explain the eventual reduction of carbon dioxide in the paleoclimatic record.
Conclusion
This post has surveyed very briefly some of the issues involved in building radiative transfer models and extending them to make predictions about climate. It is my hope that this series on infrared spectroscopy and global warming has provided some insight into the issues needed to understand climate.
Sources
- Journal of Quantitative Spectroscopy and Radiative Transfer, vol
110, pages 533-572 (2009) - Alison K. Lazarevich, Christopher C. Carter, Michael E. Thomas, Isaac N. Bankman, Eric W. Rogala, and Richard J. Green, Determination of CL Using Passive Remote Sensing of Background Radiance, Sixth Joint Conference on Standoff Detection for Chemical and Biological Defense, 25-29 October 2004, Williamsburg, VA
- Chandrasekhar, S., 1950. Radiative Transfer, Oxford University Press, London (reprinted by Dover New York).
- John M. Wallace and Peter V. Hobbs, Atmospheric Science, Second Edition, Elsevier, Amsterdam, 2006
- HITRAN
- Real Climate: A Saturated Gassy Argument
- Real Climate: What Ångström didn’t know
- Ing. R. den Blanken, Equivalent width method for quantitative FTIR analysis of biomass pyrolysis using HITRAN database, thesis, WVT 2008.10, University of Technology of Eindhoven
- NIST Webbook
- Wikipedia: Rayleigh scattering
- Wikipedia: Sunspot Number
- Wikipedia: Mt. Cleveland
- Wikipedia: Iceland Icecap
- Wikipedia: Mie Scattering




3 comments:
In my opinion the radiative transfer theory puts in the following inconsistencies when deals with planetary atmosphere.
1) It firstly contradicts the second law of thermodynamics assuming that thermal radiation (heat) flows spontaneously from a molecule at lower temperature to a molecule at higher temperature (feedback radiation).
2) Moreover one estimates the atmospheric CO2 15 microns irradiance adopting, with extreme easiness, the black body radiation laws (Plank, Stefan-Boltzmann, Kirchhoff) as if its thermal radiation is both always and however possible at any temperature.
We know very well that the radiation at 15 microns pertains to a precise vibrational resonant frequency of the CO2 molecule. On the other hand we also know that the CO2 molar heat capacity at constant volume, at atmospheric temperatures, is around 2.5*R (R is the universal gas constant) or rather that such molecules still behave in practice as rigid bodies since the collision intensity with the other molecules, due to the thermal random motion, is absolutely insufficient to start and keep up a meaningful vibrational forced oscillation and to bring it in resonance, that is the necessary condition for photon emission. But then, if there are no forced vibrational oscillations thermically rising, how can the molecules to have a thermal radiation at a vibrational frequency? Why this is possible the CO2 molar heat capacity at constant volume should be at least equal to 3.5*R, value that is reached around a temperature of 700-800 K.
With these two substantial thermodynamic inconsistencies I think that is all wrong, all to start again.
Regards.
Michele,
Thanks for your comment. You are a little confused about the second law of thermodynamics. Specifically, you are confused about the difference between kinetics and thermodynamics. Colder bodies do spontaneously radiate to hotter bodies, it's just that the hotter bodies radiate more and the average result is that the hotter body warms up the colder body. You ought to read up on rate equations and equilibrium.
Your argument about heat caopacity would seem to argue that CO2 cannot radiate, but it is easy to observe Co2 radiating. The answer is that at room temperature only about 1 in a million CO2 molecules is in v=1, vibrational motion is quantized. The effect on the heat capacity is small.
The inconsistencies you bring up are not substantial, but indicate that you have some more studying to do. Keep on doing it! But think for yourself!
I have added more substantive commentary on this topic in a post on my series on the second law: http://how-it-looks.blogspot.com/2011/07/second-law-radiative-transfer-and.html
Post a Comment