Does global warming violate the second law of thermodynamics? Such a claim may seem strange. The idea that the vast majority of physical scientists would subscribe to an idea that somehow violates a fundamental law of thermodynamics on its face seems odd. Yet, such a claim is often made by people calling the science behind global warming into question.
I prefer to call such people doubters rather than deniers. I have written a post that explains the reason for this preference. The term denier is too closely associated with Holocaust denial, and it is an unfair brand.
The term skeptic is also inappropriate because doubters rarely behave like true skeptics. In fact, the skeptical view is to follow the evidence.
To understand that evidence, we must bite off one piece at a time, and this essay focuses specifically on the erroneous claim that global warming somehow violates the second law of thermodynamics.
Radiative Transfer
Before it is possible to address this claim it is necessary to understand the context in which it is raised. For those wishing a more in-depth understanding, I have written a primer on infrared spectroscopy and global warming. Radiative transfer involves the absorption, emission, and scattering of electromagnetic radiation.
These processes in the earth's atmosphere are extremely important in understanding the energy balance of the troposphere. Carbon dioxide, in particular, absorbs infrared radiation emitted from the earth.
The carbon dioxide in each layer of the atmosphere also emits infrared radiation. This emission is dependent on the temperature of that layer; it is also dependent on the infrared spectrum of carbon dioxide, which is not a blackbody because it has discrete spectral absorption and emission.
A comment to one of the posts in that series from a person that I assume to be a student in the physical sciences in that series reads as follows:
In my opinion the radiative transfer theory puts in the following inconsistencies when deals with planetary atmosphere.
1) It firstly contradicts the second law of thermodynamics assuming that thermal radiation (heat) flows spontaneously from a molecule at lower temperature to a molecule at higher temperature (feedback radiation).
2) Moreover one estimates the atmospheric CO2 15 microns irradiance adopting, with extreme easiness, the black body radiation laws (Plank, Stefan-Boltzmann, Kirchhoff) as if its thermal radiation is both always and however possible at any temperature.
We know very well that the radiation at 15 microns pertains to a precise vibrational resonant frequency of the CO2 molecule. On the other hand we also know that the CO2 molar heat capacity at constant volume, at atmospheric temperatures, is around 2.5*R (R is the universal gas constant) or rather that such molecules still behave in practice as rigid bodies since the collision intensity with the other molecules, due to the thermal random motion, is absolutely insufficient to start and keep up a meaningful vibrational forced oscillation and to bring it in resonance, that is the necessary condition for photon emission. But then, if there are no forced vibrational oscillations thermically rising, how can the molecules to have a thermal radiation at a vibrational frequency? Why this is possible the CO2 molar heat capacity at constant volume should be at least equal to 3.5*R, value that is reached around a temperature of 700-800 K.I replied briefly and perhaps a little condescendingly to this comment as follows:
With these two substantial thermodynamic inconsistencies I think that is all wrong, all to start again.
Thanks for your comment. You are a little confused about the second law of thermodynamics. Specifically, you are confused about the difference between kinetics and thermodynamics. Colder bodies do spontaneously radiate to hotter bodies, it's just that the hotter bodies radiate more and the average result is that the hotter body warms up the colder body. You ought to read up on rate equations and equilibrium.Other than the condescending tone, there are two things I would change about my reply. The first is that I would omit the word spontaneously. It is confusing to use it in this context. Secondly, instead of referring to v=1, I should have referred to ν2 = 1. The response hits the major points, but deserves some further explanation.
Your argument about heat caopacity would seem to argue that CO2 cannot radiate, but it is easy to observe CO2 radiating. The answer is that at room temperature only about 1 in a million CO2 molecules is in v=1, vibrational motion is quantized. The effect on the heat capacity is small.
The inconsistencies you bring up are not substantial, but indicate that you have some more studying to do. Keep on doing it! But think for yourself!
The Second Law
One misunderstanding of the second law of thermodynamics is that it does not allow heat to flow from a cold body to a hot body. The person who made the comment above also misunderstands the second law of thermodynamics, but in a way that takes some explanation to understand.
The second law does prevent heat from flowing from a cold body to a hot body in a cyclic process with no other effect. See my posts on air conditioners and swamp coolers to understand why heat can be transferred from a cold body to a hot body.
Still, that is not the mistake made in this comment.
It is true that under normal (spontaneous) conditions that the net transfer of heat must be from a hot body to a cold body and not the reverse.
This fact does not mean that energy is not or cannot be transferred from the cold body to the hot body. It simply means that more energy must flow from the hot body to the cold body than vice versa.
A world in which energy could not flow from a cold body to a hot body would be very strange indeed.
Consider two gas samples of carbon dioxide at two different temperatures. Consider that the samples are placed so that infrared radiation emitted from one sample impinges on the other and vice versa.
The cold sample will emit radiation that is dependent on its temperature and its infrared spectrum. It does not somehow magically "know" that there is a hotter sample of carbon dioxide near it. It does not know that it should not emit radiation in the direction of the hotter sample.
Infrared photons that approach the hotter sample are of the correct frequencies to be absorbed by the hotter sample. The hotter sample does not magically know that the origin of these photons is a sample of carbon dioxide that is cooler than it is. It will absorb those photons in line with Beer's Law.
Now the fact is that the hotter sample of carbon dioxide radiates more photons than the cold sample. The cold sample absorbs these photons as well. The net effect is that more photons are emitted from the hot sample and absorbed by the cold sample than are emitted from the cold sample and absorbed by the hot sample.
So the net heat transfer is indeed from the hotter body to the colder body. See my post on a multi-layer model of carbon dioxide to understand this situation at a deeper level.
Why does it matter?
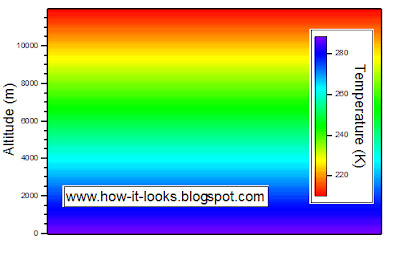
Consider the troposphere. In the troposphere, the temperature generally decreases with increased altitude. See my post on the structure of the atmosphere to understand the temperature profile of the atmosphere in more depth.
The plot below shows an idealization of the troposphere with a lapse rate of 6.5 K per 1000 meters.
An important effect of adding carbon dioxide to the troposphere is that more of the infrared radiation that originates from the earth is absorbed in the troposphere.
Therefore the troposphere warms. There are feedback mechanisms involving water that increase the effect of this warming.
The upper troposphere is still much colder than the surface of the earth. So why should a warming troposphere have any effect on surface temperature? After all, we all "know" that heat cannot be transferred from a cold body to a hot body.As explained above, the net heat does flow from hot to cold, but energy also flows from cold to hot and that energy affects the net heat flow.
Why do we use blankets to keep us warm? The trapped air, after all, is still cooler than our body temperature. In fact that trapped air keeps us warm because there is energy flow both ways.
The net effect is that heat flow is proportional to the negative gradient of the temperature. In other words, your body loses more heat outside on a cold day than it does on a warm day. There is nothing magical or counter-intuitive going on here. For a deeper understanding see my post Spontaneous Change and Equilibrium.
Vibrational Excitation
The second part of the comment may seem a bit more esoteric for the casual reader, but it does not make it any more accurate. I will try to unpack this comment a bit and explain why it is based upon an incorrect understanding.
The essence of the argument is as follows. The individual makes a back-of-the-envelope calculation of the energy content of carbon dioxide as a function of temperature. At temperatures of interest, let's say 200K - 300K to keep things simple, it is clear that the energy per molecule is not enough to excite the ν2 (~15 micron) band of carbon dioxide.
If this reasoning were correct, the vibration could never be excited at the temperatures of interest, and therefore carbon dioxide could not emit infrared radiation through the 15 micron band.
It is an empirical fact that carbon dioxide at temperatures from 200-300K does emit radiation from the 15 micron band. So where did the reasoning go wrong?
The error is that the individual who made this calculation does not sufficiently understand energy distributions in a collection of molecules. Not every molecule in a sample of carbon dioxide has the average energy of that sample. Some molecules have less energy than the average; some molecules have more energy.
The distribution of energies is well understood and is governed by Boltzmann statistics.
Back-of-the-Envelope Calculation of Average Energy
Because it is the principle that maters, not the absolute numbers, I will follow suit with a back-of-the envelope calculation without quibbling about the details.
If the constant-volume heat capacity of a mole of carbon dioxide is assumed to be about 2.5 R, then the internal energy content is about 2.5 x R x T, where R is the universal gas constant and T is the absolute temperature.
At 200 K, that is equal to about 4160 Joules/mole. At 300 K it is equal to about 6240 Joules/mole.
In a Joule of carbon dioxide, there are Avogadro's number (6.022 x 1023) of carbon dioxide molecules. So the average energy of a molecule at 200 K is 4200 divided by Avogadro's number, or 6.69 x 10-21 Joules.
At 300 K that works out to 1.04 x 10-20 Joules.
The center of the 15-micron band is at about 670 cm-1. To convert from wavenumbers to microns quickly we take 104 and divide by 670.
Now let's convert to Joules.
1 Joule(J) = 6.242 x 1018 eV = 5.034 x 1022 cm-1.
So 670 cm-1 = 670/5.034 x 1022 = 1.33 x 10-20 Joules.
So, as stated the average molecule does not have enough energy to excite the vibration.
Now consider what happens if one out of a million molecules is in the excited vibrational state. Out of a mole of molecules that would mean that 6.022 x 1017 molecules have this added energy. If we sum up all of that energy it is (1.33 x 10-20 Joules) x (6.022 x 1017 molecules), which equals 0.00801 Joules or 8.01 mJ.
Recall that a mole of carbon dioxide at 200 K has 4160 Joules and at 300 K, it has 6240 Joules.
If we subtract the energy used to excite the vibrationally excited molecules from the energy available, it has a negligible effect. In other words, the effect of the heat capacity of the vibrational excitation is negligible.
If we are worried about the heat capacity, we are free to approximate a sample of carbon dioxide as if it were made of rigid bodies, but we must not confuse an approximation with reality. Some of those molecules are vibrationally excited, a fact that can be shown by application of Boltzmann statistics.
This calculation contains many simplifications and approximations, but the exact numbers are not important. The key point to understand is that there is a distribution of energies among the molecules in a sample. Bulk properties, such as the heat capacity are not very sensitive to the fact that some of the molecules have enough energy to be in excited vibrational states.
Taking an average and concluding that none of the molecules have enough energy to be in a vibrationally excited state is a misunderstanding of the basic physics involved.
Otherwise, carbon dioxide at temperatures between 200-300 K would not thermally emit radiation via the 15 micron band.
This thermal radiation can be directly observed. If one looks at a cold sky at night, emission from water, carbon dioxide and ozone can be readily observed with passive infrared spectrometry.
A few examples of such observations are listed below.
- Infrared Emission Spectrum of the Atmosphere, Raymond Sloan, John H. Shaw and Dudley Williams, JOSA vol. 45, Issue 6, pp. 455-457 (1955)
- Upper Atmospheric Infrared Radiance from CO2 and NO Observed During the Spirit 1 Rocket Experiment, Alber-Golden, S.M.;Matthew, M.W.; Smith D.R., Journal of Geophysical Research, vol 96., Jul1. 1991 pp. 11,319 - 11,329
- Rocketborne Cryogenic (10 K) high-resolution interferometer spectrometer flight HIRIS: Auroral and Atmospheric IR Emission Spectra, Stair, A.T, Jr.; Pritchard J.; Coleman, I.; Bohne C.; Williamson, W.; Rogers, J.; Rawlins, W.T., Appl Opt. 1983, Apr 1; 22 (7), pp. 1056-1069
The Second Law Meme
I am not sure where the idea that global warming violates the second law of thermodynamics comes from. I have done some searching on Google and have found various repetitions of the claim a few examples are below:
- A post by Alan Siddons From Climate Realists
- An article by Rebecca Terrell at the New American that cites:
- A Journal article by Gerhard Gerlich and Ralf D. Tscheuschner entitled Falsification of CO2 Greenhouse Effects Within the Frame of Physics
- A post on Hockeyschtick.blogspot.com
Skepticism
Using talking points that one does not understand to dismiss science that one does not understand is not skepticism. In my post Glacier Gate and Healthy Skepticism, I discuss this issue:
A true skeptic would be as interested in refuting such a bogus claim with valid physics as he or she would be interested in challenging the claims of established climate science.Healthy skepticism involves thinking for oneself and following the trail of evidence. There is a tendency in a politicized environment to try to lead the evidence rather than follow it. In leading the evidence one takes potshots at the evidence without actually trying to understand it. A true skeptic keeps an open mind, but listens to what the evidence is saying. Claiming that the Theory of Evolution is untrue because one does not wish to believe it, is not skepticism. So it is with global warming.Neither should a true skeptic take a claim that the Himalayan glaciers will melt by 2035 at face value. A true skeptic should dig deeper and ask what such a claim is based upon. If the claim is based upon good science, one should be willing to accept it. If the IPCC researchers had dug deeper, they would have found that the prediction was premature based upon the science.
Additionally, one cannot be a skeptic of an argument that one does not understand. The right to call oneself a skeptic is earned, not asserted.
The next post in this series is entitled The Second Law, Microscopic Reversibility and Small Systems.
Sources
- Atkins, P. W. Physical Chemistry, W. H. Freeman and Company, New York, 3rd edition, 1986
- McQuarrie, Donal d A., Statistical Thermodynamics, University Science Books, Mill Valley, CA, 1973
- Bromberg, J. Philip, Physical Chemistry, Allan and Bacon, Inc., Boston, 2nd Edition, 1984
- Infrared Emission Spectrum of the Atmosphere, Raymond Sloan, John H. Shaw and Dudley Williams, JOSA vol. 45, Issue 6, pp. 455-457 (1955)
- Upper Atmospheric Infrared Radiance from CO2 and NO Observed During the Spirit 1 Rocket Experiment, Alber-Golden, S.M.;Matthew, M.W.; Smith D.R., Journal of Geophysical Research, vol 96., Jul1. 1991 pp. 11,319 - 11,329
- Rocketborne Cryogenic (10 K) high-resolution interferometer spectrometer flight HIRIS: Auroral and Atmospheric IR Emission Spectra, Stair, A.T, Jr.; Pritchard J.; Coleman, I.; Bohne C.; Williamson, W.; Rogers, J.; Rawlins, W.T., Appl Opt. 1983, Apr 1; 22 (7), pp. 1056-1069
- NIST WebBook
- A post by Alan Siddons From Climate Realists
- An article by Rebecca Terrell at the New American
- A Journal article by Gerhard Gerlich and Ralf D. Tscheuschner entitled Falsification of CO2 Greenhouse Effects Within the Frame of Physics
Contents
- Introduction
- What the Second Law Does Not Say
- What the Second Law Does Say
- Entropy is Not a Measure of Disorder
- Reversible Processes
- The Carnot Cycle
- The Definition of Entropy
- Perpetual Motion
- The Hydrogen Economy
- Heat Can Be Transferred From a Cold Body to a Hot Body: The Air Conditioner
- The Second Law and Swamp Coolers
- Entropy and Statistical Thermodynamics
- Fluctuations
- Partition Functions
- Entropy and Information Theory
- The Second Law and Creationism
- Entropy as Religious, Spiritual, or Self-Help Metaphor
- Free Energy
- Spontaneous Change and Equilibrium
- The Second Law, Radiative Transfer, and Global Warming
- The Second Law, Microscopic Reversibility, and Small Systems
- The Arrow of Time
- The Heat Death of the Universe
- Gravity and Entropy
- The Second Law and Nietzsche's Eternal Recurrence
- Conclusion





6 comments:
I stumbled on this looking for properties of CO2 at 100K.
There is a touch more to the situation than discussed here.
The radiant properties of CO2 are a perfect example of the second law. At higher temperature and higher density the probability of escape increases while return decreases. The radiant impact of more CO2 in the upper atmosphere requires convection to transport the energy needed and the return of cooler air with convection to warmer the surface. If the average temperature of the air falling to replace convection is greater due to CO2 then the surface can warm, due to CO2 enhancement.
Sorry, I cannot parse what you are trying to say. If you are simply pointing out that convection has a role to play, you are absolutely correct. The point of my post, however, is that radiant energy flows both ways with a net transport along the gradient.
You are wrong because you have not addressed the situation in which the emissivity of one body is very different from another.
You cannot explain the Second Law if the hotter body emits less than the cooler body because the hotter one has lower emissivity, now can you? Net radiation in that case goes from cold to hot.
You need to read Prof Claes Johnson's "Computational Blackbody Radiation" and/or my 'radiation' page at http://climate-change-theory.com
The temperature information is contained in the value of the peak frequency of the radiation. So a warmer surface can, and does, merely scatter radiation from a cooler atmosphere without converting it to thermal energy.
Hence the greenhouse conjecture is a physical impossibility.
In more detail ...
The frequency distribution for a cooler emitter (the atmosphere) is fully contained within the frequency distribution of an emitter at a higher temperature (the surface), and it is shifted towards lower frequencies because the peak frequency is proportinal to the (absolute) temperature.
Hence any radiation from the cooler emitter can potentially resonate with the warmer emitter and be re-emitted immediately without any conversion to thermal energy. This is what Prof Claes Johnson has proved does happen and it is the only plausible explanation for the veracity of the Second Law of Thermodynamics applying to radiation.
In contrast, the frequency distribution of the warmer emitter does extend into higher frequencies than that of the cooler emitter. The area between the curves in this region gives a measure of the probability of conversion of such radiation to thermal energy. Thus this probability is nil until there is a temperature difference, and then increases as the temperature difference increases.
We see that virtually all solar radiation reaching the surface (UV, visible and IR) will be converted to thermal energy because there is virtually no overlap between the spectra of incident solar radiation and that of emitted radiation from the surface.
Hence the surface absorbs solar radiation, converting it to thermal energy which can subsequently exit the surface layer (usually at night, but even months later in local winter) by diffusion, conduction, evaporation, radiation and chemical processes, followed by convection once the energy is into the air. There is subsequently negligible probability of any of that energy returning to the surface or remaining in the surface layer of the atmosphere for longer than it would otherwise have done just because of carbon dioxide, methane or similar trace gases.
Mr. Cotton,
Thanks for your comment. Your thoughts about emissivity misunderstand physics, but in a very interesting way.
Consider two bodies A and B. A is colder than A, but has near unit emissivity at all wavelengths. B is hotter but has a very small, but non-zero emissivity in the region of interest. It is very possible for the brightness temperature of the colder body to be higher than the warmer body.
Aha, you say, that means more photons are being emitted from the cold body than the hotter body, and so the net flux must violate the second law.
That is not correct. Because B has a low emissivity, it also has a low absorptivity, therefor it does not efficiently absorb the photons coming its way, and the second law is not violated.
Some of your other comments deeply misunderstand molecular spectroscopy. I suggest a good starting place is here: http://how-it-looks.blogspot.com/2010/01/primer-on-infrared-spectroscopy-and.html
All this greenhouse gases stuff is great, however humans only contribute 0.28% of all greenhouse gases which is a very tiny amount.
Mr. Marsh,
Interesting assertion. I suspect you are confused by the fact that most of the gas in the atomsphere is nitrogen and oxygen, but as shown on my blog, these are not Greenhouse gases. Human beings are without question responsible for the rapid increase in Carbon Dioxide, which is the elephant in the room because of its high concentration relative to other greenhouse gases except water in the troposphere). Clearly, humans are not responsible for water in the troposhere, but increased heat from warming that arises from carbon dioxide increases the amount of water vapor in the air.
Post a Comment