- A Hoax
- Not Dipole Allowed
- Degenerate
- A Blackbody
- The reason the sky is blue
- Even though I understand Global Warming, I don't know
- I don't know
Topics
9/11
Acquisition Reform
Advertising
Alaway
Alcohol
Ale
Allergies
Antisemitism
Barack H. Obama
Beer
Billiards
Biology
Books
Budget
Bureaucracy
California
Capitalism
Carbohydrates
Carcinogen
CDC
Chemical Warfare
Chemistry
Chemophobia
Chirality
Climate Science
Colonial Pines
Computers
Conservation Laws
Constitution
Consumerism
Cosmology
CPT Invariance
Creationism
Customer Service
Daesh
David Irving
Dead End
Defense
Dinosaurs
Disasters
Economic
Energy
English
Ethics
Evolution
Fluoride
Food
FTL
Garden Care
George W. Bush
Gerlich and Tscheuschner
GISS
Glaciers
GMOs
HadCRU
Haiti
Health
Himalayan Rock Salt
HITRAN
Holocaust Denial
Home Brewing
How It Looks From Here
html
Humor
Information
Infrared Spectroscopy
IPCC
Iran
ISIS
Islam
Islamophobia
Israel
Ketotifen Fumarate
Law
Lawn Care
Leibniz
Lisbon
Magnetism
Math
Medco
Medicine
Modeling
Molecules
Monopoly
Monsanto
Naphazoline hydrochloride
Neutrinos
Nietzsche
NIH
NIST
Noether's Theorem
Non-hazardous
Norton Ghost
Nuclear Warfare
Oil
Oil Spill
Olopatadine hydrochloride
Opinion
Orson Scott Card
Parody
Pataday
Patanol
Pesticides
Pheneramine maleate
Physics
Plumbing
Politics
Poll
Pope
POTUS
Prescriptions
Prop 65
Psychology
Quantum Mechanics
Quiz
Racism
Radiative Transfer
Relativity
Religion
Respiration
Senior Housing
Signs
Smoking
Specific Gravity
Statistics
Stock Market
Sugars
Sun Tzu
Surface Temperature
Surgeon General
Symantec
Target
Temperature
Terrorism
The Final Solution
The Holocaust History Project
Thermodynamics
Time
Trains
Units
Voltaire
von Clausewitz
Weather
White House
Wine
Yeast
Friday, December 10, 2010
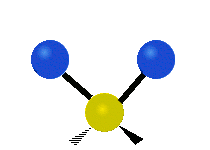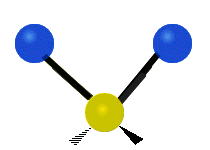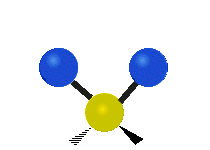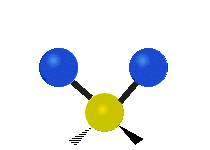
Carbon Dioxide Poll
A recently closed poll on this blog posed the following question. "The bending mode of carbon dioxide is:" Respondents were to respond with the best answer. Here were the choices:
Friday, November 12, 2010
Fluctuations
This post is part of a series,Nonsense and the Second Law of Thermodynamics The previous post is entitled Entropy and Statistical Dynamics.
The second law of thermodynamics works because of the statistics of very large numbers. Consider a bouncing ball: as it bounces, it dissipates heat and eventually does not bounce as high.

The second law of thermodynamics works because of the statistics of very large numbers. Consider a bouncing ball: as it bounces, it dissipates heat and eventually does not bounce as high.

Saturday, November 6, 2010
Entropy and Statistical Thermodynamics
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled The Second Law and Swamp Coolers.
A previous post discusses the macroscopic thermodynamic definition of entropy, but there is another, statistical way of describing entropy. Consider an isolated macroscopic system of interacting molecules. Without knowing much about what is going on with the individual molecules, it is possible to measure macroscopic thermodynamic properties such as the pressure, the temperature etc.
(Figure Source)
Consider that the system is isolated; so that the total energy of the entire system of molecules is a constant. Energy is free to move from one molecule to another, and each molecule has multiple electronic, vibrational, rotational, and translational energy states that it could be in. There are many distinguishable ways that the system could be arranged to achieve the this energy.
A previous post discusses the macroscopic thermodynamic definition of entropy, but there is another, statistical way of describing entropy. Consider an isolated macroscopic system of interacting molecules. Without knowing much about what is going on with the individual molecules, it is possible to measure macroscopic thermodynamic properties such as the pressure, the temperature etc.
Consider that the system is isolated; so that the total energy of the entire system of molecules is a constant. Energy is free to move from one molecule to another, and each molecule has multiple electronic, vibrational, rotational, and translational energy states that it could be in. There are many distinguishable ways that the system could be arranged to achieve the this energy.
Saturday, October 30, 2010
The Second Law and Swamp Coolers
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled Heat Can Be Transferred From a Cold Body to a Hot Body: The Air Conditioner.
When I was an undergraduate, I had a physical chemistry professor who claimed that air conditioners that were completely inside a room could not possibly work. Opening the refrigerator door on a hot day will not make your house cooler.
The refrigerator gives off more heat than it transfers from inside itself; a refrigerator is actually heating the house. If the door is left open, the refrigerator works harder to try to maintain a cool temperature in accordance with its thermostat setting. As the refrigerator works harder, it releases more heat into the house.
My professor, however, was not correct. It is possible to have a cooling unit that does more cooling than it releases heat to the environment. The trick with indoor coolers is that the process by which they operate is not cyclic.
When I was an undergraduate, I had a physical chemistry professor who claimed that air conditioners that were completely inside a room could not possibly work. Opening the refrigerator door on a hot day will not make your house cooler.
The refrigerator gives off more heat than it transfers from inside itself; a refrigerator is actually heating the house. If the door is left open, the refrigerator works harder to try to maintain a cool temperature in accordance with its thermostat setting. As the refrigerator works harder, it releases more heat into the house.
My professor, however, was not correct. It is possible to have a cooling unit that does more cooling than it releases heat to the environment. The trick with indoor coolers is that the process by which they operate is not cyclic.
Tuesday, October 26, 2010
Heat Can Be Transferred From a Cold Body to a Hot Body: The Air Conditioner
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled The Hydrogen Economy.
As of 10/26/2010, a survey on this site shows that 25% (Final result 21%) of the respondents thus far think that the second law of thermodynamics says that heat cannot be transferred from a cold body to a hot body.
Not only are these people mistaken, but they are also ignoring their own common experience of the world.
As of 10/26/2010, a survey on this site shows that 25% (Final result 21%) of the respondents thus far think that the second law of thermodynamics says that heat cannot be transferred from a cold body to a hot body.
Not only are these people mistaken, but they are also ignoring their own common experience of the world.
It is possible to transfer heat from a cold reservoir to a hot reservoir!
Friday, October 15, 2010
The Hydrogen Economy
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled Perpetual Motion.
The media often perpetuate the idea that the so-called hydrogen economy is the solution to all of our energy needs. Hydrogen is abundant everywhere; in fact there are oceans full of hydrogen in the form of water, just waiting to be extracted, oxidized and used as an endless source of energy, right?
The media often perpetuate the idea that the so-called hydrogen economy is the solution to all of our energy needs. Hydrogen is abundant everywhere; in fact there are oceans full of hydrogen in the form of water, just waiting to be extracted, oxidized and used as an endless source of energy, right?
Monday, October 11, 2010
Perpetual Motion
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled The Definition of Entropy.
It is a consequence of conservation of energy and the second law of thermodynamics that it is impossible to build a perpetual motion machine. There are many types of proposed perpetual motion machines.
There is a post that goes into a lot of detail of the various sorts of perpetual motion machines by Kevin T. Kilty, entitled Perpetual Motion. Rather than go into arcane detail about different types of perpetual motion machines, I think it suffices to refer the interested reader to Kilty's post.
No machine can generate more more energy than put in (first law of thermodyanics, conservation of energy, Noether's theorem). The first law of thermodynamics states that work can be converted into heat, and heat can be converted into work, but that the sum, the so-called internal energy (E or U) is a conserved quantity.
It is a consequence of conservation of energy and the second law of thermodynamics that it is impossible to build a perpetual motion machine. There are many types of proposed perpetual motion machines.
There is a post that goes into a lot of detail of the various sorts of perpetual motion machines by Kevin T. Kilty, entitled Perpetual Motion. Rather than go into arcane detail about different types of perpetual motion machines, I think it suffices to refer the interested reader to Kilty's post.
No machine can generate more more energy than put in (first law of thermodyanics, conservation of energy, Noether's theorem). The first law of thermodynamics states that work can be converted into heat, and heat can be converted into work, but that the sum, the so-called internal energy (E or U) is a conserved quantity.
Saturday, October 9, 2010
The Definition of Entropy
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled: The Carnot Cycle. This post is heavily dependent on the previous post; so I recommend reading it first.
Let q represent the heat transferred in a process, and qrev represent the heat transferred in a reversible process. Let T be the absolute temperature (in Kelvin).
The sum of qrev/T for all steps of the process over a full Carnot cycle is equal to zero. In fact, it is true for any reversible cyclic process.
Let q represent the heat transferred in a process, and qrev represent the heat transferred in a reversible process. Let T be the absolute temperature (in Kelvin).
The sum of qrev/T for all steps of the process over a full Carnot cycle is equal to zero. In fact, it is true for any reversible cyclic process.
Labels:
Chemistry,
Energy,
Physics,
Temperature,
Thermodynamics,
Units
Friday, October 8, 2010
The Carnot Cycle
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled: Reversible Processes.
In 1824, Nicolas Léonard Sadi Carnot tried to explain how heat could be converted into useful work. He came up with a four-step cycle that is known as the Carnot cycle.
In 1824, Nicolas Léonard Sadi Carnot tried to explain how heat could be converted into useful work. He came up with a four-step cycle that is known as the Carnot cycle.
Sunday, October 3, 2010
Reversible Processes
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled: Entropy is Not a Measure of Disorder.
To understand the macroscopic thermodynamic definition of entropy, it is important to understand something called a reversible process. A reversible process is just what it sounds like: a process that is reversible.
A reversible process should be thought of as an ideal case. In a reversible process, the system is in equilibrium for every infinitesimal step of the process. Imagine a balloon filled with gas, and imagine that the balloon is perfect, i.e., we need not concern ourselves with the properties of the balloon itself: we care only about the gas inside the balloon and the gas outside the balloon.
At equilibrium, the pressure on each side of the balloon is equal. If the pressure outside of the balloon is reduced, the balloon expands until the pressures are equal again. In a reversible process, the balloon is allowed to expand continuously by infinitesimal steps. The reversible process acts as a limit to any real process.
To understand the macroscopic thermodynamic definition of entropy, it is important to understand something called a reversible process. A reversible process is just what it sounds like: a process that is reversible.
A reversible process should be thought of as an ideal case. In a reversible process, the system is in equilibrium for every infinitesimal step of the process. Imagine a balloon filled with gas, and imagine that the balloon is perfect, i.e., we need not concern ourselves with the properties of the balloon itself: we care only about the gas inside the balloon and the gas outside the balloon.
At equilibrium, the pressure on each side of the balloon is equal. If the pressure outside of the balloon is reduced, the balloon expands until the pressures are equal again. In a reversible process, the balloon is allowed to expand continuously by infinitesimal steps. The reversible process acts as a limit to any real process.
Friday, October 1, 2010
Entropy Is Not a Measure of Disorder
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled: What the Second Law Does Say.
Entropy is not a measure of disorder. Entropy is not a measure of disorder.
To paraphrase Stanford Professor H.C. Anderson, there are a lot of sentences in the English language that contain the words "entropy" and "disorder," and most of them are wrong. There are many reputable text books and sources that say that entropy is disorder; nevertheless, entropy is not a measure of disorder.
Entropy is not a measure of disorder. Entropy is not a measure of disorder.
To paraphrase Stanford Professor H.C. Anderson, there are a lot of sentences in the English language that contain the words "entropy" and "disorder," and most of them are wrong. There are many reputable text books and sources that say that entropy is disorder; nevertheless, entropy is not a measure of disorder.
Monday, September 27, 2010
What the Second Law Does Say
This post is part of a series, Nonsense and the Second Law of Thermodynamics. The previous post is entitled: What the Second Law Does Not Say.
There are multiple valid ways to state the second law of thermodynamics. Some ways of expressing the law do so in terms of macroscopic notions such as heat and temperature.
Other descriptions employ the concept of entropy, which is based upon a statistical approach to thermodynamics. Some alternative macroscopic statements include:
There are multiple valid ways to state the second law of thermodynamics. Some ways of expressing the law do so in terms of macroscopic notions such as heat and temperature.
Other descriptions employ the concept of entropy, which is based upon a statistical approach to thermodynamics. Some alternative macroscopic statements include:
- There can be no process with the sole result of absorbing heat and completely converting it into work.
- It is impossible to convert heat completely into work in a cyclic process.
- It is impossible to carry out a cyclic process using an engine connected to two heat reservoirs that will have as its only effect the transfer of a quantity of heat from the low-temperature reservoir to the high-temperature reservoir.
Labels:
Chemistry,
Energy,
Physics,
Temperature,
Thermodynamics
Saturday, September 25, 2010
What the Second Law Does Not Say
This post is part of a series, Nonsense and the Second Law of Thermodynamics.
The Second Law does not say it is impossible for heat to be transferred from a cold body to a hot body. The second law does not say that "disorder" must increase on the earth or anywhere else. Life is not a counter-example to the second law; life is an example of the second law in action.
One has to be very careful about applying statistical results to a single molecule or a few molecules and remembering that increasing entropy applies to irreversible changes, not reversible ones. The second law says nothing about disorder. The second law does not prevent evaporative coolers from operating.
The second law does not contradict radiative transfer theory or global warming. The second law does not contradict conservation of energy. In applying the second law to cosmology, one should tread cautiously.
The Second Law does not say it is impossible for heat to be transferred from a cold body to a hot body. The second law does not say that "disorder" must increase on the earth or anywhere else. Life is not a counter-example to the second law; life is an example of the second law in action.
One has to be very careful about applying statistical results to a single molecule or a few molecules and remembering that increasing entropy applies to irreversible changes, not reversible ones. The second law says nothing about disorder. The second law does not prevent evaporative coolers from operating.
The second law does not contradict radiative transfer theory or global warming. The second law does not contradict conservation of energy. In applying the second law to cosmology, one should tread cautiously.
Nonsense and the Second Law of Thermodynamics
Introduction
The Second Law of Thermodynamics is, perhaps, the most abused physical law of all time. It may be rivaled for that distinction by the Uncertainty Principle, Relativity, and Hawking Radiation, but I think the Second Law probably wins the contest.
There is a plethora of nonsense disseminated on the web and elsewhere that misrepresents what the law actually says. This series is an attempt to curb some of that nonsense. Along the way, I hope to make some sense of what the second law of thermodynamics actually does say, as well as addressing some of the nonsense that people believe about it.
The Second Law of Thermodynamics is, perhaps, the most abused physical law of all time. It may be rivaled for that distinction by the Uncertainty Principle, Relativity, and Hawking Radiation, but I think the Second Law probably wins the contest.
There is a plethora of nonsense disseminated on the web and elsewhere that misrepresents what the law actually says. This series is an attempt to curb some of that nonsense. Along the way, I hope to make some sense of what the second law of thermodynamics actually does say, as well as addressing some of the nonsense that people believe about it.
Wednesday, September 1, 2010
Reformatted Post on Beer's Law
I have had to reformat the post on Beer's Law. The html editor at Blogspot seems to do strange things with <br> tags.
Thursday, August 12, 2010
Bush on Islam
"The face of terror is not the true faith of Islam. That's not what Islam is all about. Islam is peace. These terrorists don't represent peace. They represent evil and war. When we think of Islam we think of a faith that brings comfort to a billion people around the world. Billions of people find comfort and solace and peace. And that's made brothers and sisters out of every race -- out of every race. America counts millions of Muslims amongst our citizens, and Muslims make an incredibly valuable contribution to our country. Muslims are doctors, lawyers, law professors, members of the military, entrepreneurs, shopkeepers, moms and dads. And they need to be treated with respect. In our anger and emotion, our fellow Americans must treat each other with respect."
- George W. Bush 17 September 2001
- George W. Bush 17 September 2001
Labels:
9/11,
George W. Bush,
Islam,
Islamophobia,
Politics,
Terrorism
Sunday, August 8, 2010
Very Unscientific Poll
I have posted a poll on global warming on this blog. Before I remove the poll from the blog, I thought I should document the results. The poll is for entertainment purposes only. There was no methodology used except to post the poll to this blog using a blogger add-on.
The poll states "Global Warming is," and gives six options, "anthropogenic," "natural," "a hoax," "something I don't understand," "a topic upon which I am reserving judgment," and "other."
The poll states "Global Warming is," and gives six options, "anthropogenic," "natural," "a hoax," "something I don't understand," "a topic upon which I am reserving judgment," and "other."
Friday, July 30, 2010
How To Convert To and From Parts-Per-Million (ppm)
Parts-per million (ppm) is a common quantity used in many areas of math and science. It can be somewhat difficult for some to understand because it is not a true unit. In fact, ppm is a unitless quantity. It is analogous to percent. Percent can refer to just about anything. Percent means part-per-hundred, per cent (cent meaning hundred). ppm is an exactly analogous quantity, but it is one part per million instead of one part per hundred. ppm is a ratio between two numbers that have the same units. Consider the example of a 5% sales tax. For every hundred dollars I spend, I must pay 5 dollars in sales tax. So:
5 dollars/100 dollars = 5%
I would much rather pay a 5 ppm sales tax:
5 dollars/106 dollars = 5 ppm
We do not usually refer to money in ppm, but we could. Ppm is more often found as a concentration, for example, ppm by mass or ppm by volume (sometimes referred to as ppmv). In nuclear magnetic resonance spectroscopy ppm can be used to describe the amount of chemical shift in frequency (Hz/MHz). This post focuses on the use of ppm as a measure of concentration.
5 dollars/100 dollars = 5%
I would much rather pay a 5 ppm sales tax:
5 dollars/106 dollars = 5 ppm
We do not usually refer to money in ppm, but we could. Ppm is more often found as a concentration, for example, ppm by mass or ppm by volume (sometimes referred to as ppmv). In nuclear magnetic resonance spectroscopy ppm can be used to describe the amount of chemical shift in frequency (Hz/MHz). This post focuses on the use of ppm as a measure of concentration.
Friday, July 23, 2010
The Medco Model of Customer Service
I am enrolled with a health plan through which I get my medications from Medco®. On June 25, my doctor sent in a prescription for Patanol®, an allergy medication for the eyes. As of July 10, I had received no word on the prescription.
On Saturday, July 10, I called Medco to find out what had happened. After navigating their inane voice-mail system, I was able to speak to a human being. I achieved this amazing feat by repeatedly saying "representative" to all of the voice-mail prompts.
The woman on the other end told me that my prescription had stopped for some unknown reason, but that she could restart it manually and that I should expect it to arrive in 8-10 days. She told me that I could go to my local pharmacy and that Medco would pay for a 7-day supply to hold me over. It sounded too good to be true.
Saturday, July 17, 2010
A Sucker Is Born Every Day!
Snake-oil salesmen are alive and well. People will buy nonsense if it is marketed correctly. I enjoyed the review of the wine clip on the Dan's Data Website. This device supposedly makes wine better by pouring the liquid through a magnetic field. The review does a good job of explaining the nonsense.
Source
Source
- Dan's Data: Dan's Data: Review of the wine clip
Friday, July 9, 2010
How To Convert To and From Wavenumbers
The question of how to convert from one set of units to another comes up from time-to-time, and I think it might be helpful to have a few short posts that simply address unit conversion. This post addresses conversion to and from wavenumbers (cm-1) (also called reciprocal centimeters, inverse centimeters or Kaisers). A previous post What is Infrared Radiation (IR)? addresses the concepts behind this unit. The unit is proportional to frequency, and can be considered a unit of frequency or of energy.
Friday, June 25, 2010
Thinking About Oil Rain
Recently, there has been discussion on the Internet of a video that purportedly shows evidence of it raining oil in Louisiana.
Is this evidence of oil rain? Is oil rain possible?
Is this evidence of oil rain? Is oil rain possible?
Radiative Transfer
If you are following this primer on infrared spectroscopy and global warming you already have some of the basics of radiative transfer. The previous post in this series develops a simple multi-layer model of the carbon dioxide in the troposphere. It leaves out many important features but shows conceptually how absorption and emission behave in layers of the troposphere.
The current post is intended to wrap up the topic and touch upon a few issues that were not discussed. It is possible to teach a year-long course in radiative transfer (or even multiple courses); so of course this post does not do the topic justice, but perhaps it provides some basic principles that give the reader a cursory understanding of the topic.
The current post is intended to wrap up the topic and touch upon a few issues that were not discussed. It is possible to teach a year-long course in radiative transfer (or even multiple courses); so of course this post does not do the topic justice, but perhaps it provides some basic principles that give the reader a cursory understanding of the topic.
Friday, June 4, 2010
Chemical Free Universal Weed Killer
***AMAZING GREEN WEEDKILLER***
* UNIVERSAL: WORKS ON ALL WEEDS *
!!!WILL NOT HARM LAWN OR GARDEN!!!
(WHEN USED AS DIRECTED)
$$ SAVE MONEY $$
Cheaper than Harmful Chemicals!
SINGLE PURCHASE WILL LAST YEARS FOR LESS THAN THE COST OF CONVENTIONAL WEEDKILLERS!
**IT'S FERROMAGNETIC!!**
Thursday, May 20, 2010
A Multi-Layer Model of Carbon Dioxide
I have put together a simple multi-layer model of carbon dioxide in the troposphere. It is based upon the same principles as the two-layer model and the three-layer model. It accounts for the temperature and pressure profiles from the previous post and it is part of a primer on infrared spectroscopy and global warming. Just like those other models there are still caveats; this model is intended to be illustrative of concepts and therefore it is conceptually simple.
Saturday, May 15, 2010
Structure of the Atmosphere
This post is part of a primer on infrared spectroscopy and global warming. The previous post introduces a three-layer model in the context of developing a radiative-transfer model of the atmosphere. From that post it should be apparent that one needs to include an understanding of the structure of the atmosphere to understand radiative transfer through the atmosphere. This post provide a summary of the structure of the atmosphere. It is intended to be a quick introduction, rather than a detailed treatise. Some of the sources listed go into more detail for the interested reader.
Saturday, April 17, 2010
A Three-Layer Model
This post is part of a primer on infrared spectroscopy and global warming. The previous post introduces a two-layer model and is a necessary prerequisite to understanding this post. In this post I start with the following assumptions. There is a source of infrared radiance that has emissivity of 1, i.e., it radiates as a perfect blackbody at a temperature of 288 K. The radiance from that layer is I0
There is a layer of air 1000 m thick with 380 ppm carbon dioxide at a temperature of 278 K. There is another layer of air 1000 m thick with 380 ppm carbon dioxide at 268 K. All layers are at a constant pressure of one atmosphere.
There is a layer of air 1000 m thick with 380 ppm carbon dioxide at a temperature of 278 K. There is another layer of air 1000 m thick with 380 ppm carbon dioxide at 268 K. All layers are at a constant pressure of one atmosphere.
Friday, April 9, 2010
A Two-Layer Model
This post is part of a primer on infrared spectroscopy and global warming. The previous post discusses the issue of saturation in the 14-micron band of carbon dioxide in a single-layer model. The post before that discusses Beer's Law, and is a necessary prerequisite to understanding this post. This post starts to look beyond the single-layer model, by discussing a two-layer model, and beginning a discussion of radiative transfer.
Friday, April 2, 2010
A Note On Saturation of the Carbon Dioxide 15-micron Band
This post is part of a primer on infrared spectroscopy and global warming. The previous post discusses Beer's Law, and is a necessary prerequisite to understanding this post. The previous post also introduced the idea of saturation of a single layer model. This post looks more deeply at the single-layer model and the saturation of the 15-micron band of carbon dioxide.
Saturday, March 6, 2010
Infrared Spectra of Molecules of Interest
This post is part of a primer on infrared spectroscopy and global warming. The previous post concludes a three-post series that looks at molecules and radiation and discusses how molecules give rise to infrared spectra. This post discusses the infrared spectra of some molecules of interest.
Figure Source
Figure Source
Friday, February 26, 2010
Molecules and Radiation III: Vibration, Dipoles, and Ro-Vibrational Spectra
This post is part of a primer on infrared spectroscopy and global warming. The previous post looked at the vibrational modes of several molecules including HCl and several molecules of atmospheric interest. This post discusses how these modes relate to infrared absorption and uses HCl as an example.
Friday, February 19, 2010
OPINION: Don't Call Climate Doubters Deniers
I am friendly with and love people who doubt the reality of climate change. The same cannot be said for those who "doubt" the reality of the Final Solution. Lately it has become commonplace to refer to the former as "deniers," but the implicit connection to the latter offends me. To understand why, I think it is useful to look at these groups of people.
Friday, February 12, 2010
Molecules and Radiation II: Molecular Vibration, Rotation, and Translation
This post is part of a primer on infrared spectroscopy and global warming. The previous post starts the process of looking at the interaction between infrared radiation and molecules and discusses the degrees of freedom of molecules and the Born-Oppenheimer approximation. The result of the previous post is that for the purposes of IR spectroscopy, one can focus on the motion of the atomic nuclei and separate them from the electronic degrees of freedom of a molecule.
Figure Source
There are 3N nuclear degrees of freedom for a molecule that has N nuclei. For the HCl molecule that means there are 6 degrees of freedom.
Figure Source
There are 3N nuclear degrees of freedom for a molecule that has N nuclei. For the HCl molecule that means there are 6 degrees of freedom.
Wednesday, February 10, 2010
Does Secondhand Smoke Kill?
By now everyone knows that smoking can kill, but does secondhand smoke really kill and how do we know that?
The National Institute of Health (NIH) has a good resource on Secondhand Smoke, and it is perhaps a good place to start. The NIH starts with a very general description from the National Cancer Institute:
The National Institute of Health (NIH) has a good resource on Secondhand Smoke, and it is perhaps a good place to start. The NIH starts with a very general description from the National Cancer Institute:
You don't have to be a smoker for smoking to harm you. You can also have health problems from breathing in other people's smoke. Secondhand smoke is the combination of smoke that comes from the burning end of a cigarette, cigar or pipe and the smoke exhaled by the smoker. Secondhand smoke contains more than 50 substances that can cause cancer. Health effects of exposure to secondhand smoke include lung cancer, nasal sinus cancer, respiratory tract infections and heart disease. There is no safe amount of secondhand smoke. Children, pregnant women, older people and people with heart or breathing problems should be especially careful.
Sunday, February 7, 2010
Molecules and Radiation I: Molecular Structure
This post is part of a primer on infrared spectroscopy and global warming. The previous post starts the process of looking at the interaction between infrared radiation and matter and discusses black-bodies and relationship between temperature and infrared radiation. This post goes further and looks at how gas phase molecules interact with infrared radiation.
For a molecule to absorb radiation, several conditions must hold. First, energy must be conserved: if the molecule absorbs energy from a photon, the molecule must be able to store that energy in some manner.
Saturday, January 30, 2010
Infrared Radiation, Black-bodies, and Temperature
This post is part of a primer on infrared spectroscopy and global warming. The previous post discusses the nature of infrared radiation. This post starts the process of looking at the interaction between infrared radiation and matter and discusses black-bodies and the relationship between temperature and infrared radiation.
Saturday, January 23, 2010
What is Infrared Radiation (IR)?
This post is part of a primer on infrared spectroscopy and global warming. The main post gives an overview of the topic and provides links to each of the sections. This post examines what infrared radiation (IR) is, a necessary first step to understanding the importance of IR in discussions about global warming.
Figure source
IR is a type of electromagnetic radiation; so the starting place is to understand electromagnetic radiation.
Figure source
IR is a type of electromagnetic radiation; so the starting place is to understand electromagnetic radiation.
Friday, January 22, 2010
"Glacier-Gate" and Healthy Skepticism
The last report of the The Intergovernmental Panel on Climate Change (IPCC) contained the claim that there was a probability that the glaciers in the Himalayas would melt by 2035. It now appears that such a claim never should have been made. It is instructive to review the bidding to see how this claim appeared in the report.
The claim stated:
The claim stated:
Glaciers in the Himalaya are receding faster than in any other part of the world (see Table 10.9) and, if the present rate continues, the likelihood of them disappearing by the year 2035 and perhaps sooner is very high if the Earth keeps warming at the current rate. Its total area will likely shrink from the present 500,000 to 100,000 km2 by the year 2035 (WWF, 2005).First, I provide some background on the IPCC. Second, I discuss the source of this claim, and third, I discuss what this error means. My conclusion is a discussion of healthy skepticism and what it means. Those who claim that the IPCC made an error and therefore climate science is a hoax, are not healthy skeptics of the sort I mean.
Friday, January 15, 2010
Voltaire's Poem on the Lisbon Disaster (1756)
The link below has the poem and also some discussion of its context. the poem is a reply to those like Pope and Leibniz who proclaimed that all is well.
"UNHAPPY mortals! Dark and mourning earth!
Affrighted gathering of human kind!
Eternal lingering of useless pain!
Come, ye philosophers, who cry, "All’s well,"
And contemplate this ruin of a world."
http://courses.essex.ac.uk/cs/cs101/volt/Lisbon2.htm
"UNHAPPY mortals! Dark and mourning earth!
Affrighted gathering of human kind!
Eternal lingering of useless pain!
Come, ye philosophers, who cry, "All’s well,"
And contemplate this ruin of a world."
http://courses.essex.ac.uk/cs/cs101/volt/Lisbon2.htm
Thursday, January 14, 2010
The Beer-Lambert Law
Introduction
This post is part of a primer on infrared spectroscopy and global warming. The previous post looks at the features of the spectra of molecules of interest molecules and radiation and discusses how molecules give rise to infrared spectra. This post looks at the question of how much radiation is absorbed by gas phase molecules in a laboratory setting and examines some of the differences between the laboratory gas cell and the earth's atmosphere.
This post is part of a primer on infrared spectroscopy and global warming. The previous post looks at the features of the spectra of molecules of interest molecules and radiation and discusses how molecules give rise to infrared spectra. This post looks at the question of how much radiation is absorbed by gas phase molecules in a laboratory setting and examines some of the differences between the laboratory gas cell and the earth's atmosphere.
Wednesday, January 13, 2010
Please Consider Donating to Help the Haitian People
By now everyone is aware of the devastating earthquake it Haiti and its aftermath. 10s or perhaps 100s of thousand of people have died. More will die without aid. The Haitian people need your help, not just now, but in the weeks and months to come. There are many options to help.

You can donate to the American Red Cross International fund online. You can even make your payment through Amazon.

You can donate to the American Red Cross International fund online. You can even make your payment through Amazon.
Tuesday, January 12, 2010
Monday, January 11, 2010
The Past Tense of To Be
The past tense of the verb to be is not conjugated as follows:
I was
you was
it was
we was
you was
they was
I was
you was
it was
we was
you was
they was
Sunday, January 10, 2010
A Primer on Infrared Spectroscopy and Global Warming
Introduction
This post and the posts linked to it through section headers together form a primer on infrared spectroscopy and how it relates to global warming. The purpose of the primer is not be to convince skeptics that global warming is real, but rather to explain some of the terms and issues being discussed in climate science. My goal is not to write a super technical explanation of infrared spectroscopy. That's been done so many times that it is hardly worth doing again.
Rather, my intent is to write something that clearly describes infrared spectroscopy and relates it to global warming that tries to explain some fairly technical concepts in reasonably plain language. As such there is an inevitable loss of fidelity about some of the fine points of infrared spectroscopy. Anyone interested in such detail can follow some of the sources that I will provide. At some point one has to compromise between accessibility and technical accuracy. I hope that the choices made in this primer are helpful to some people trying to understand this topic. This post is an outline of the topics addressed in the linked entries.
What is Infrared Radiation (IR)?
Figure source
This first post starts with the basics. It discusses the electromagnetic spectrum and where infrared radiation fits into it. It discusses waves and their measures. It introduces the idea of electric dipole radiation. It discusses the units of radiation wavelength, frequency. It ends by discussing photons and energy quantization.
Saturday, January 9, 2010
Getting the Blog into Shape
Just a note to say that I am getting this new blog into shape. I welcome any advice on format, readability etc. Please let me know if you like the look and feel and whether you find it easy to read. Also, if there are any topics you'd like me to get into, I'd welcome suggestions.
Friday, January 8, 2010
The Chemistry of Holocaust Denial
This post is an opportunity to collect in one place some of the articles I've written on Holocaust Denial. I have not been actively involved with this topic in years. The deniers may have additional pseudo-arguments of which I am not aware, but I think as a whole the articles stand.
In 2001, I wrote an Expert Report to support the defense of Deborah Lipstadt and Penguin Books against the libel accusations of David Irving.
Irving appealed the decision and Lipstadt won again.
In 2001, I wrote an Expert Report to support the defense of Deborah Lipstadt and Penguin Books against the libel accusations of David Irving.
David Irving had sued Deborah Lipstadt in the UK for libel because of the fact that she identified him as a Holocaust denier, Hitler apologist, distorter of history, antisemite and racist in her book Denying the Holocaust. It should be noted that Lipstadt did not choose the legal system as the place to fight this battle. By suing her for libel, Irving threatened her right of free speech as well as the accuracy of the historical record. As a US citizen, Lipstadt could have conveniently ignored the suit and relied on the protections of the US First Amendment. Instead, she courageously rose to the challenge, deciding that UK legal precedent is of historical importance. She defended herself using the defense of justification. Her book was not libel because it was true. She won the suit, Mr. Justice Gray found that the defense of justification was in fact a valid one.
Irving appealed the decision and Lipstadt won again.
The Claim of a Warming Pause
Introduction
There are many claims that global warming has stopped. Such claims look at the average of the surface temperature record over the past few years and conclude that ever since 1998 temperatures have cooled.
The trick is that 1998 was a particularly warm year. In one of the commonly used data sets, 1998 was the hottest year ever recorded. If 1998 was so hot and all of the years since 1998 have been cooler, does that mean that the earth has cooled since 1998?
The fact is that one cannot demonstrate a trend by starting with one year and comparing each year since that year individually. There are two problems with such an analysis. First, if the analysis always starts with 1998, the data are being cherry-picked.
Why start with 1998? Why not start with 2000, or 1995? If someone starts with 1998, it is because he or she is trying to use the data to support a pre-determined conclusion. If one is interested in understanding what information is really embedded in the data, one cannot pick and choose where to start. Of course one has to work with the data that are available. It would be nice to go back to the Medieval Warm Period and plant sensors all over the earth, but one cannot do such a thing.
There are many claims that global warming has stopped. Such claims look at the average of the surface temperature record over the past few years and conclude that ever since 1998 temperatures have cooled.
The trick is that 1998 was a particularly warm year. In one of the commonly used data sets, 1998 was the hottest year ever recorded. If 1998 was so hot and all of the years since 1998 have been cooler, does that mean that the earth has cooled since 1998?
The fact is that one cannot demonstrate a trend by starting with one year and comparing each year since that year individually. There are two problems with such an analysis. First, if the analysis always starts with 1998, the data are being cherry-picked.
Why start with 1998? Why not start with 2000, or 1995? If someone starts with 1998, it is because he or she is trying to use the data to support a pre-determined conclusion. If one is interested in understanding what information is really embedded in the data, one cannot pick and choose where to start. Of course one has to work with the data that are available. It would be nice to go back to the Medieval Warm Period and plant sensors all over the earth, but one cannot do such a thing.
How It Looks From Here
I am going to experiment with setting up my own blog. I am motivated to do so because of my experience with Facebook. I only recently joined Facebook and have enjoyed catching up with friends, family and associates. I seem to have two different goals when I am on Facebook. The first is personal: I want to interact with my friends and share personal tidbits.
Facebook is well suited for this type interaction. Secondly, I like to share my perspective on numerous topics of interest to me. Facebook is not so well suited for this usage. I often have more to say than is conveniently displayed in a Facebook note. Moreover some of my friends and associates probably are more interested in what is going on with me personally than my views on such topics. So I am going to experiment with blog posting for a little while. Whether I continue this exercise will depend on how much of a time sink it is, and whether anyone is actually interested in reading it.
Topics that I will probably discuss include thoughts on Antisemitism, Acquisition Reform, Climate Science, Evolution, The Final Solution, Holocaust Denial, Human Rights, Humanism, Humor, Infrared Spectroscopy, Judaism, Management, Math, Military, News, Public Policy, etc.
At the moment, I am interested in Climate Science, its popularization, and its mis-characterization. Some of my initial posts will probably relate to this topic. I am an unapologetic fan of Real Climate. Some of the content there is a bit technical and I hope that I can help make it accessible. Additionally, I have a bit of experience with passive infrared spectroscopy. It seems to me that climate science presents a teachable moment on infrared spectroscopy and I'm interested in pursuing that possibility.
In the past I have been very interested in Holocaust Denial and I have been a contributor to The Holocaust History Project. From time to time I will probably post on the topic.
Facebook is well suited for this type interaction. Secondly, I like to share my perspective on numerous topics of interest to me. Facebook is not so well suited for this usage. I often have more to say than is conveniently displayed in a Facebook note. Moreover some of my friends and associates probably are more interested in what is going on with me personally than my views on such topics. So I am going to experiment with blog posting for a little while. Whether I continue this exercise will depend on how much of a time sink it is, and whether anyone is actually interested in reading it.
Topics that I will probably discuss include thoughts on Antisemitism, Acquisition Reform, Climate Science, Evolution, The Final Solution, Holocaust Denial, Human Rights, Humanism, Humor, Infrared Spectroscopy, Judaism, Management, Math, Military, News, Public Policy, etc.
At the moment, I am interested in Climate Science, its popularization, and its mis-characterization. Some of my initial posts will probably relate to this topic. I am an unapologetic fan of Real Climate. Some of the content there is a bit technical and I hope that I can help make it accessible. Additionally, I have a bit of experience with passive infrared spectroscopy. It seems to me that climate science presents a teachable moment on infrared spectroscopy and I'm interested in pursuing that possibility.
In the past I have been very interested in Holocaust Denial and I have been a contributor to The Holocaust History Project. From time to time I will probably post on the topic.
Subscribe to:
Comments (Atom)
























 I
I


